
Interceptor® Plus (milbemycin oxime/praziquantel)
Interceptor Plus is a monthly, tasty chew that prevents heartworm disease and treats and controls adult hookworm, roundworm, whipworm and tapeworm infections in dogs and puppies 6 weeks of age and older and 2 pounds of body weight or greater.
Defend Your Patients With 5-Worm Protection
Interceptor Plus is indicated for the treatment of 5 different types of worms, protecting dogs and puppies against heartworm disease, adult hookworm, roundworm, tapeworm and whipworm infections.
- HEARTWORM
(Dirofilaria immitis)
- ROUNDWORM
(Toxocara canis and Toxascaris leonina)
- ADULT TAPEWORM
(Taenia pisiformis, Echinococcus multilocularis, Echinococcus granulosus and Dipylidium caninum)
- WHIPWORM
(Trichuris vulpis)
- HOOKWORM
(Ancylostoma caninum)
The Whipworm Advantage: Protected Against the Undetected
Whipworms and tapeworms are commonly undetected in float tests, allowing them to continue spreading and are the most common internal parasites found in dogs.
- 96% of D. caninum infections were missed using passive flotation1
- 62% of T. vulpis infections were missed using passive flotation2
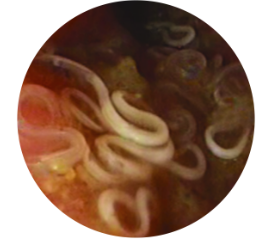
Parasite Prevalence in Your Practice Area3:
Why Interceptor Plus Stands Apart:
Based on label comparisons
*Interceptor Plus protects against Ancylostoma caninum
†Heartgard Plus protects against Ancylostoma caninum, Uncinaria stenocephala, Ancylostoma braziliense
‡Proheart 12 protects against Ancylostoma caninum and Uncinaria stenocephala
Interceptor Plus Product Information
For the prevention of heartworm disease, and the treatment and control of adult roundworm, adult hookworm, adult whipworm, and adult tapeworm infections.
Chewable
milbemycin oxime/praziquantel
Interceptor Plus Dosing and Administration
| Product - Milbemycin Oxime (per tablet) |
| 2-8 lbs - 2.3 mg |
| 8.1-25 lbs
- 5.75 mg |
| 25.1 - 50 lbs
- 11.5 mg |
| 50.1 - 100 lbs
- 23 mg |
| 100+ lbs
- Administer the appropriate combination of chewable tablets. |
| Product - Praziquantel (per tablet) |
| 2-8 lbs - 22.8 mg |
| 8.1-25 lbs
- 57 mg |
| 25.1 - 50 lbs
- 114 mg |
| 50.1 - 100 lbs
- 228 mg |
| 100+ lbs
- Administer the appropriate combination of chewable tablets. |
| Product | 2-8 lbs | 8.1-25 lbs | 25.1 - 50 lbs | 50.1 - 100 lbs | 100+ lbs |
|---|---|---|---|---|---|
| Milbemycin Oxime (per tablet) | 2.3 mg | 5.75 mg | 11.5 mg | 23 mg | Administer the appropriate combination of chewable tablets. |
| Praziquantel (per tablet) | 22.8 mg | 57 mg | 114 mg | 228 mg | Administer the appropriate combination of chewable tablets. |
How To Administer Interceptor Plus
 1. Remove 1 Tasty Chewable from its Packaging
1. Remove 1 Tasty Chewable from its PackagingCheck to make sure that the chewable has not expired and remove from packaging.
 2. Administer Chewable
2. Administer ChewableThe dog can take the chewable from the hand or with a small amount of food. Make sure it consumes the whole dose.
 3. Check for Complete Ingestion
3. Check for Complete IngestionAfter the dog has taken the chewable, observe it for a few minutes to ensure that no part of the dose is lost or rejected. If you suspect that any of the dose has been lost, redosing is recommended.
Why choose Interceptor Plus?
Protection against heartworm disease, adult hookworm, roundworm, tapeworm and whipworm infections.
- Single monthly chew for easy administration.
- Interceptor Plus comes in 4 convenient dosing sizes.

Don’t Leave a Gap in Their Coverage
Interceptor Plus is a once-monthly flavored chew that protects against all 5 major types of dangerous worms in dogs: heartworm, hookworm, roundworm, whipworm and tapeworm.
Effective and Proven Success: Heartworm Disease Prevention
In a controlled laboratory study, Interceptor Plus was 100% effective against induced heartworm infections when administered once-monthly for six consecutive months.

Interceptor Plus Combines Two Powerful Ingredients
- Milbemycin oxime prevents heartworm disease in dogs and treats and controls adult hookworm, roundworm and whipworm infections.
- Praziquantel has demonstrated 100% efficacy in controlled studies against four species of adult tapeworms.⁴
Interceptor® Plus (milbemycin oxime/praziquantel) Resources
Indications for Interceptor® Plus
Interceptor® Plus is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment and control of adult roundworm (Toxocara canis, Toxascaris leonina), adult hookworm (Ancylostoma caninum), adult whipworm (Trichuris vulpis), and adult tapeworm (Taenia pisiformis, Echinococcus multilocularis, Echinococcus granulosus and Dipylidium caninum) infections in dogs and puppies 6 weeks or older and 2 pounds or greater.
Important Safety Information
Treatment with fewer than 6 monthly doses after the last exposure to mosquitoes may not provide complete heartworm prevention. Prior to administration of Interceptor® Plus, dogs should be tested for existing heartworm infections. The safety of Interceptor® Plus has not been evaluated in dogs used for breeding or in lactating females. The following adverse reactions have been reported in dogs after administration of milbemycin oxime or praziquantel: vomiting, diarrhea, depression/lethargy, ataxia, anorexia, convulsions, weakness, and salivation. For full prescribing information see the Interceptor® Plus package insert.














